中文


01 Application of BIM Technology
The BIM Technology Center of the company is of rich practical experience in terms of BIM model building, in-depth design of construction drawings, pipeline collision inspection and assistance in on-site installation with the help of BIM software. It applies the BIM technology to the process implementation management of large-scale projects, such as assistance in the statistical management of materials, cost forecast, construction simulation, plan determination and processing of fabricated parts, playing a positive role in project quality improvement.

Mechanical room 360
panoramic display

Technical interlayer
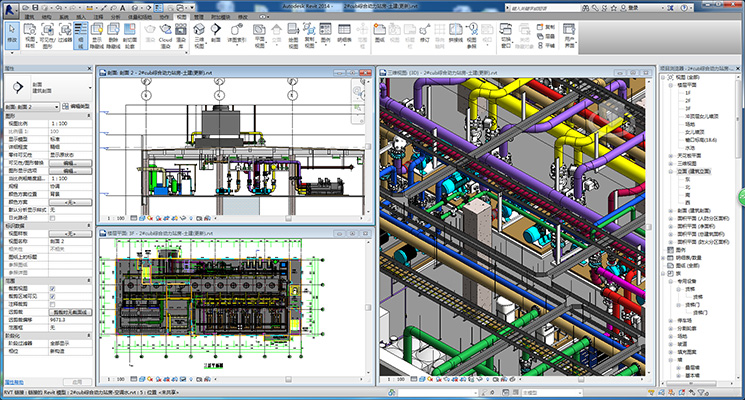
Impact detection and
deepening design



02 Application of CFD Air Flow Distribution Simulation Technology
With the rise of computer simulation technology, the simulation technology of air flow in cleanrooms has become an emerging frontier technology in the engineering construction field.
With the help of CFD simulation software, the company's CFD technical team simulates various air flow distributions and temperature and humidity fields in the cleanroom under the static environment and dynamic environment, which has achieved phased results. At the same time, it also provides technical support for the company's marketing and HVAC design.

Simulation of room pressure field

Room temperature field simulation
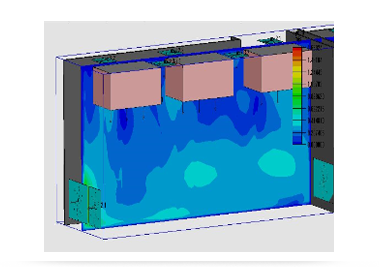
Cloud image simulation of airflow velocity
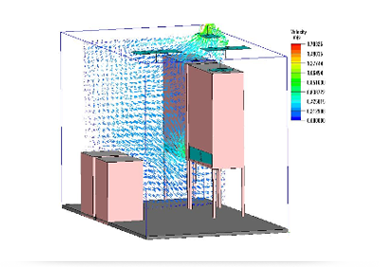
Simulation of airflow velocity trajectory diagram
03 Application of GMP Validation Technology
GMP validation is an important link in the process from project completion to obtaining the operation and production license. With the comprehensive implementation of the new versions of GMP and GSP quality standards certification in China, the trend of increasingly strict drug regulation in China is irreversible, and it is increasingly difficult for pharmaceutical factories to pass GMP validation. In order to better serve pharmaceutical projects of clean engineering, the company has set up a GMP validation center to provide validation services during the implementation of pharmaceutical projects, which can complete the validation of various projects and ensure their smooth pass of GMP validation.
400 892 1298
苏ICP备15056857号-1 Enterprise Post Office
COPYRIGHT©ALLRIGHTSRESERVED.China Electronics System Engineering No. 2 Construction Co., Ltd.
Business Scope
Corporate Culture
Party Building





